An Evaporation Crystallization Process of the Type Described in
The wet filter cake consists of solid K2SO4crystals and a 400 wt K2SO4solution is a ratio 10 kg crystalskg solution. Freon-113 C2Cl3F3 has an enthalpy of vaporization of 270 kJmol and a normal boiling point of 480 C.

Description Of An Evaporative Crystallization Process With Recycle Youtube
The wet filter cake consists of solid K2SO4crystals and a 400 wt K2SO4solution is a ratio 10 kg crystalskg solution.

. The wet filter cake consists of solid K2SO4 crystals and a 400 wt K2SO4 solution in a ratio 10 kg crystalskg solution. An evaporation-crystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt. The wet filter cake consists of solid K SO crystals and a 400 wt K SO solution in a ratio 10 kg crystalskg solution.
An evaporationcrystallization process of the type described is used to obtain solid potassium sulfate from an aqueous solution of this salt. An evaporationcrystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt. An evaporation crystallization process is used to obtain solid potassium sulphate crystal K250a from an aqueous solutio Q.
An evaporation-crystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt. An evaporationcrystallization process of the type described in Example 452 is used to obtain solid potassium sulfate from an aqueous solution of this salt. Crystals and a 400 wt K2SO4 solution in a ratio 10 kg crystalskg solution.
The wet filter cake consists of solid K 2 SO 4 crystals and a 400 wt K 2 SO 4 solution in a ratio 10 kg crystalskg. An evaporation-crystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqeuous solution of this salt. An evaporationcrystallization process of the type described in Example 452 is used to obtain solid potassium sulfate from an aqueous solution of this salt.
An evaporation-crystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt. The fresh feed to the process contains 196 wt K 2 SO 4. The wet filter cake consists of solid K2SO4 crystals and a 400wt K2SO4 solution in a ratio 10 kg crystalskg solution.
The fresh feed to the process contains 196 wt K2SO4. An evaporation-crystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt. The fresh feed to the process contains 196 wt K 2 SO 4.
The fresh feed to the process contains 196 wt K2SO4. The wet filter cake consists of solid K2SO4 crystals and a 400 wt K2SO4 solution is a ratio 10 kg crystalskg solution. The wet filter cake consists of solid K2SO4 crystals and a 400 wt K2SO4 solution in a ratio 10 kg crystalskg solution.
The fresh feed to the process contains 196 wt K 2 SO 4. An evaporationcrystallization process of the type described in Example 452 is used to obtain solid potassium sulfate from an aqueous solution of this salt. The fresh feed to the process contains 196 wt K 2 S O 4 mathrmK_2 mathrmSO_4 K 2 SO 4.
The wet filter cake consists of solid K_2SO_4 crystals and a 400 wt K_2SO_4 solution in a ratio 10 kg crystalskg solution. The filtrate also a 400. The wet filter cake consists of solid K 2 SO 4 crystals and a 400 wt K 2 SO 4 solution in a ratio 10 kg crystalskg solution.
The fresh feed to the process contains 196 wt K SO. The fresh feed to the process contains 196 wt K2SO4. The wet filter cake consists of solid K2SO4 crystals and a 400 wt K2SO4 solution in a ratio 10 kg crystalskg solution.
The wet filter cake consists of solid K2SO. The fresh feed to the process. The filtrate also a 400 solution is.
This process can be carried out by causing a physical change like a change in temperature or a chemical change like acidity. Crystallization is a natural process which happens when the materials solidify from a liquid or as they precipitate out of a liquid or gas. Evaporation-crystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt.
The wet filter cake consists of solid K2SO4 crystals and a 400 wt K2SO4 solution in a ratio 10 kg crystalskg solution. The fresh feed to the process contains 196 wt K_2SO_4. The fresh feed to the process contains 196 wt K2SO4.
The fresh feed to the process contains 196 wt K2SO4. The fresh feed to the process contains 196 wt K 2 SO 4. An evaporationcrystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt.
An evaporationcrystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt. An evaporation-crystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt. The wet filter cake consists of solid K2SO4 crystals and a 400 wt K2SO4 solution in a ratio 10 kg crystalskg solution.
Evaporation-crystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt. An evaporation-crystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt. The fresh feed to the process contains 196wt K2SO4.
The wet filter cake consists of solid K 2 SO 4 crystals and a 400 wt K 2 SO 4 solution in a ratio 10 kg crystalskg solution. The crystallization process is carried out on the basis of the size and shapes. The wet filter cake consists of solid K_SO.
The fresh feed to the process contains 196 wt K2SO4. The fresh feed to the process contains 196 wt K2SO4. An evaporationcrystallization process of the type described in Example 452 is used to obtain solid potassium sulfate from an aqueous solution of this salt.
The fresh feed to the process contains 196 wt K2SO4. The fresh feed to the process contains 196 wt K2SO4. The fresh feed to the process contains 196 wt mathrmK_2 mathrmSO_4.
Crystals and a 400 wt K2SO4 solution in a ratio 10 kg crystalskg solution. The wet filter cake consists of solid K2SO4 crystals and a 400 wt K2SO4 solution in a ratio 10 kg crystalskg solution. An evaporation-crystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt.
An evaporation-crystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt. The fresh feed to the process contains 196 wt K2SO4. Question 3 45 marks An evaporation-crystallization process of the type described in Example 45-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt.

An Evaporation Crystallization Process Of The Type Described In Example 4 5 2 Is Used To Obtain Solid Brainly Com
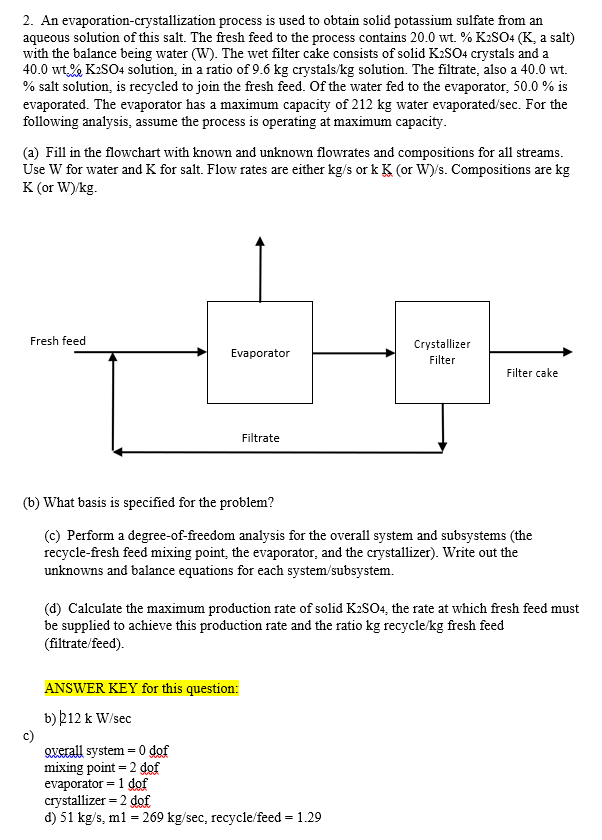
Solved An Evaporation Crystallization Process Is Used To Chegg Com
Solved Frb 4 45 An Evaporation Crystallization Process Of The Type Course Hero
Comments
Post a Comment